Utilization of Natural Isolates of Saccharomyces cerevisiae Strains for Production of Valuable Chemicals
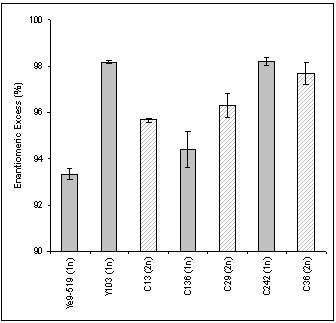
The biotransformation of 4-chloro-acetoacetic acid ethyl ester (CAAE) to 4-chloro-3-hydroxybutanoic acid ethyl ester (CHB), using S. cerevisiae, is a well studied model reaction for the asymmetric reduction of ?-keto esters to their corresponding ?-hydroxy esters. However, previous studies using non-modified S. cerevisiae for S-CHB production reported poor enantioselectivity with a maximum enantiomeric excess (ee) of 92% when the minimum value required for industrial use is >95% ee. Some of the natural S. cerevisiae strains, isolated from Mount Carmel National Park in Israel, were characterized as resistant to environmental stress. Nevertheless, these strains showed relatively low ee values, while a laboratory strain, Y103, exhibited a selectivity of 98% ee.
The aim of this project is to obtain a robust strain adapted for industrial production of chiral compounds by crossing an enantioselective strain and a multi-stress resistant strain.
A further objective is to screen for a robust yeast strain capable of producing 2-phenylethanol (PEA), an important flavor and fragrance compound with a rose-like odor. Although the vast majority of PEA is produced chemically, the natural production is preferred, when applied in the food industry. The public demand for natural flavors and economical considerations, motivate the design of highly PEA producing S. cerevisiae strains.
Enantioselectivity of the yeast strains studied. Parents (Y103-selective, Ye9-519-stress tolerant) were crossed to obtain a stress-tolerant and enantioselective haploid offspring (C242) (Nir et al., AMB, 2008)