Modulating selectivity of FAD-dependent monooxygenases
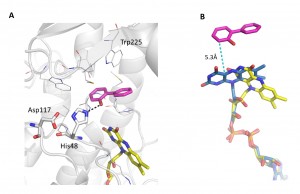
2-Hydroxybiphenyl 3-monooxygenase (HbpA) is an FAD dependent monooxygenase which catalyzes the ortho-hydroxylation of a broad range of substrates in the presence of NADH and molecular oxygen. We have determined the structure of HbpA from the soil bacterium Pseudomonas azelaica HBP1 with bound 2-hydroxybiphenyl, as well as several variants, at a resolution of 2.3-2.5 Å to investigate structure function correlations of the enzyme.
A crystal structure of HbpA with bound 2-hydroxybiphenyl in the active site