Engineering lipase T6 for improved stability in methanol
Lipases are beneficial for the synthesis of biodiesel. They enable the use of low grade feedstocks comprising free fatty acids and water, they operate under mild reaction conditions, and facilitate better downstream processing compared with the chemical equivalent. Nevertheless, their low stability in methanol, a substrate for the biodiesel transesterification reaction, hampers their utilization in industrial processes. We used protein engineering methods to generate a lipase T6 variant with improved stability in methanol.
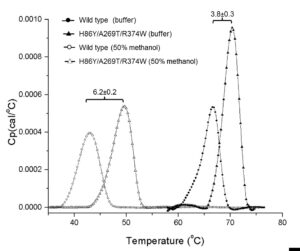
The triple mutant, H86Y/A269T/R374W, had a half-life value at 70% methanol of 324 min which reflects an 87-fold enhanced stability compared to the wild type together with elevated thermostability in buffer and in 50% methanol. This variant also exhibited an improved biodiesel yield from waste chicken oil compared to commercial Lipolase 100L® and Novozyme® CALB. Crystal structures of the wild type and the methanol-stable variants provided insights regarding structure-stability correlations. The most prominent features were the extensive formation of new hydrogen bonds between surface residues directly or mediated by structural water molecules, and the stabilization of Zn and Ca binding sites.
This project is funded by the Israel Ministry of Environmental Protection
Increase in thermal unfolding of lipase T6 variant H86Y/A269T/R374W as a measure of its improved stability in methanol