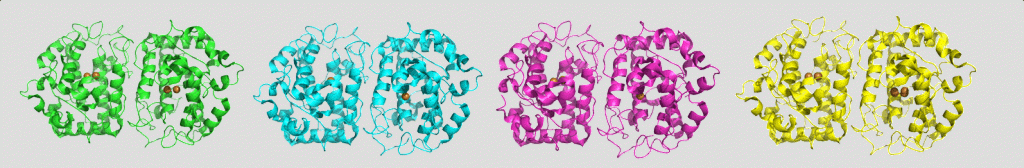Tyrosinase, a ubiquitous enzyme in all domains of life, performs two successive oxidation reactions: conversion of monophenols to diphenols, and oxidation of diphenols to quinones, subsequently turning them into melanin. Our group solved the structure of a bacterial tyrosinase in the presence of the monophenol tyrosine, and the diphenol L-Dopa. The structures prove unequivocally that all of the substrates are stabilized in the same orientation at the active site of the enzyme, and are positioned towards the same copper ion, unlike previous assumptions. Understanding of the mechanism and structure of tyrosinase can help in developing inhibitors for treatment of hyperpigmentation and age spots, improving the sensitivity of melanoma cells towards radiation therapy, and prevention of browning of fruits and vegetables.