Studying Structure-Function Correlations of Tyrosinase
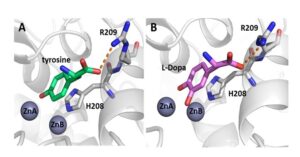
Tyrosinases are copper-containing enzymes which are ubiquitously distributed in the animal, plant and fungal kingdoms, and to a lesser extent, in bacteria. Molecular oxygen is used by tyrosinases to catalyze two different enzymatic reactions: (i) the ortho-hydroxylation of monophenols to o-diphenols (monophenolase activity) and (ii) the oxidation of o-diphenols to o-quinones (diphenolase activity). The aim of this research is to study structure function correlations of tryosinase from Bacillus megaterium. We have crystallized the enzyme with bound tyrosine and DOPA, proving that both monophenols and diphenols bind similarly in the active site.
This project is funded by the Israel Science Foundation
Tyrosinase from Bacillus megaterium with bound substrates in the active site